— Topics —
Calorie
2023.02.17
Calorie Calculation: Why the Atwater Coefficient Is Not Perfect
-
Contents
-
- What is the Atwater coefficient in the first place?
- Digestion is an entirely different process than burning food
(I)~(V) - Perspective from my intestinal starvation theory
<Conclusions>
Many experts still believe that “one calorie is one calorie.” If we apply this idea to weight control, we have to only be concerned with the number of calories in our diet, regardless of what different foods we eat or how we eat them. Of course, the human body is not that simple, and many researchers have warned against this kind of thinking.
In explaining this, I thought I should make a distinction between reactions that occur "inside" the body (after absorption) and those that occur “outside" the body (before absorption) (*1).
In this article, I would like to consider the Atwater coefficient, which is the basis for calorie labels on food products, as an issue that occurs outside the body.

As many gut microbiologists recognize, the digestive organs such as the stomach and intestines are "external" to the body (e.g. bad bacteria in the gut do not directly harm the body), which is perfectly consistent with what I have been explaining in this blog-the idea that the "absorption rate is important."
( *1: "Diet-induced heat production" actually occurs after absorption, but it is related to digestion, so I’d like to also use it here to explain it as an external response.)
1. What is the Atwater coefficient in the first place?
In the 1800’s, chemists developed a method to measure the amount of calories in food by burning food and measuring the temperature change from its surroundings.
Burning food is chemically similar to the process by which our bodies break down food to obtain energy.
Much of what we know about food calories is said to come from the research in the late 19th century by Wilbur Atwater at Wesleyan University in Connecticut, who conducted a variety of experiments aimed at understanding human metabolism and the energy content of different foods.

By feeding volunteers various foods and calculating the difference in the heat of combustion between the food and the excreta, he approximated the calories absorbed by his volunteers. It is said that Atwater also took into account the dietary fiber which we can’t digest (*2), and proteins, some of which are excreted in the urine as urea after being absorbed.
More than one-hundred-twenty years after this experiment, these "Atwater coefficient" are still the basis for caloric calculations for all foods[1].
( *2: It is now known that dietary fiber produces some energy through fermentation and breakdown by the bacteria in the large intestine[2].)

Currently, the general Atwater coefficients of 4kcal/g for carbohydrate and protein, 9kcal/g for fat, and 7kcal/g for alcohol are applied to all foods regardless of the type of food.
The use of specific Atwater coefficient is also allowed, which is different for each food group[3].
2. Digestion is an entirely different process than burning food
We ingest food and then break down complex food molecules into simple structures such as glucose and amino acids by various digestive enzymes. After that, we get an energy source by absorbing them. Naturally, this is a completely different process compared to burning food in the laboratory.
According to Professor Rob Dunn (North Carolina State University), every calorie count on food labels is based on estimates or approximations, and are not an accurate reflection.
Recent research has revealed that how many calories we get from a given food depends on a variety of factors, including which species we eat, how we cook food, which bacteria are in our gut, and how much energy we use to digest different foods (diet-induced heat production).[4]
(I)Digestibility varies even in vegetables
Even if it is same vegetable group, they vary in the firmness of their leaves and stems. The cell walls of plant cells in the stems and leaves of some species are tough, whereas those of spinach, cucumber, and lettuce, etc. are soft and more than 90% of them are water.
Also, even in the same plant, the durability of cell walls can differ. The older the leaves, the tougher the cell walls tend to be and the more difficult they are to digest.
Seeds such as corn and nuts, in particular, have such sturdy cell walls that they can hoard precious calories within them and pass through the body intact[4].

A study by Janet A. Novotny at the U.S. Department of Agriculture (2012) found that when people eat almonds, they take in just 129 kcal per serving, not the 170 kcal listed on the label. It is beginning to be proven that nuts such as peanuts, almonds, and walnuts have a more robust cellular structure than other foods with similar levels of energy sources, and that their cell walls limit digestion. It is possible that the Atwater coefficient overestimates the digestibility of nuts.[5]
(II)Calories vary depending on how we cook food
Prof. Dunn also mentions that the biggest problem with modern calorie labels is that they failed to account for how food is prepared and processed, which dramatically changes the amount of energy derived from food.
We humans learned to cook raw food. We learned to process foods in different ways such as simmering, baking, frying, or even fermenting, to make them more palatable and tender.
This should have dramatically increased the calories we extracted from food [4].

Furthermore, some have pointed out that industrial food processing not only exposes food to high temperatures and pressures, but also softens food by adding air, to make it even easier to get more calories.
Corn, for instance, which is considered indigestible, is made into potage, and raw peanuts are roasted and processed into peanut butter. These processes must have dramatically increased the amount of energy available to the body. In other words, not all pork dishes are the same. The energy used for digestion and the absorption rate differ when roasting a chunk of pork versus making it into pâté.
(III)Energy required for digestion and immunity
Some research has shown that the energy required for digestion is not the same. This is called “diet-induced thermogenesis,” and it requires a great deal of energy to convert proteins into amino acids, fats into fatty acids, and carbohydrates into glucose. It is said that when proteins are broken down into amino acids, they require much more energy to digest than fats, because enzymes must unravel their tightly wound bonds[6].
It also differs between whole grains and refined wheat. A 2010 study found that people who ate 600-or 800-kcal portions of whole-wheat bread with sunflower seeds, kernels of grain, and cheddar cheese, expended twice as much energy to digest that food as those who ate the same amount of white bread and "processed cheese products.” Consequently, those who ate whole-wheat bread substantially obtained ten percent fewer calories, they said[7].

Many Japanese and Koreans traditionally love to eat raw fish or meat, if they’re fresh.
However, raw meat, for example, has been found to harbor many dangerous microbes, and our immune systems use energy to identify and deal with those pathogens[4].
It is possible that the same amount of cooked meat takes less energy to digest than steak tartare and has more usable calories .
(IV)Differences in digestive enzymes and intestinal bacteria
Most babies have lactase, an enzyme necessary to break down lactose sugar in milk, but it is said that most adults don’t produce this enzyme.
It has also been found that when starches such as rice and spaghetti are left to cool after being cooked, some of these starches crystallize into structures that digestive enzymes cannot easily break down.
What’s more, some microbes are present only in specific ethnic groups. Some Japanese, for example, have a microbe in their intestines which is suitable for breaking down seaweed. It has been found that these intestinal bacterium stole the seaweed-digesting genes from a marine bacterium that lingered in raw seaweed [4].
(V)There are variations for the method of calculation
The general Atwater coefficients were calculated based on the average daily diet of Americans at the time. Digestibility for carbohydrates, fats, and proteins were assumed to be 97, 95, and 92 percent respectively, and after adjusting a little for this, protein and carbohydrates were set at 4kcal/g, fats at 9kcal/g, and alcohol at 7kcal/g[8]. Although metabolizable energy values vary slightly for proteins, depending on whether they are vegetable or animal protein, and for carbohydrates, depending on whether they are sugar or starch, the coefficients were derived by a system of an average.
On the other hand, specific Atwater coefficients are also allowed, which divides food into several groups and applies a representative coefficient of that group to the entire group.
It is said that the U.S. Food and Drug Administration (FDA) allows a total of five variations on the theme including these, and some point out that depending on the method chosen by the food company, there may be variation on the calorie labels[9]. When such uncertainties add up, the daily caloric intake could vary widely.
<Summary of this section>
Prof. Dunn mentions as follow:
(1) It is possible to modify the Atwater system for every food group, as in the almond example. However, this would require a challenge to reexamine the amount of nutrients retained in the excreta for every food.
(2) However, even if we completely revised calorie counts, they would never be accurate because how many calories we extract from food depends on a complex interaction between food, the human body, and its many microbes. In particular, the process of digestion is so complex that it is probably impossible to derive an accurate formula for calorie calculation that will suit everyone.
(3) Instead, we should think more carefully about the energy we get from food in the context of human biology. Processed foods are easily digested in the stomach and intestines, and thus provide a lot of energy for very little work. On the other hand, vegetables, nuts, and whole grains require more sweat to digest, offer far more vitamins and nutrients than processed foods, and keep our gut bacteria happy[4].
3. Perspective from my intestinal starvation theory
I believe that researchers and nutritionists at that time, including Atwater, were committed to ensuring that people could have an adequate amount of nutrition, and the calorie counting system they created had great merit. But I suspect it has been misperceived by some and is now causing problems with those who are overweight.
The reason why the issue of being overweight remains unresolved indefinitely is because many people are too fixated on the caloric value of foods.
Let's say you eat, as in the example in the section 2-[III], 400kcal of a meal: whole-wheat bread, nuts, and grilled chicken. Assuming that, after taking into account the energy required for digestion, you obtained ten percent fewer calories (360kcal), the argument that "wouldn't it be the same if you ate 360 kcal worth of white bread and chicken terrine?" is complete nonsense.

Fibers from whole-wheat bread and nuts tend to remain undigested in our intestines, which means there is a message to the body of "there is still food," but the combination of white bread and easily digestible protein, etc. is quickly digested, and if the "three factors + one" of my theory are met, the intestinal starvation message saying "there’s no food" would be sent to the brain through the small intestine.
In other words, you can gain weight despite a reduction in total daily caloric intake.
I I have been explaining throughout this blog that the fundamental difference between obese people and lean people can be explained by the difference in set-point weight, and that having a higher set-point weight is related to an increase in absorption efficiency, which is induced by intestinal starvation. In addition, since one of the key factors causing intestinal starvation is how fast you digest food, both digestion and absorption ability are extremely important in my theory.
Nevertheless, if we believe that only numbers based on averages of subjects are all we have, we ignore them. As Prof. Dunn mentions, I don’t believe that the complexity of digestion and absorption for a diverse population can be described by a system using an average.
(Please read the following article for other issues in "calorie counting.")
There is No Meaning in Simply Calculating Calories You Consume
Conclusions
The Atwater coefficients is a measure of how much energy we can obtain from food, but I think it is inadequate to address the problem of obesity. Even if we revised the Atwater coefficients more accurately by taking into account energy required for digestion and food composition, the obesity problem will not be solved if we judge things only by the number of calories.
One idea that has been suggested by some researchers is to introduce a "traffic-light" system on food labels, alerting consumers to foods that are highly processed (red dots), lightly processed (green dots) or in-between (yellow dots) [10], and I agree with this idea. It is also possible to combine satiety, the number of chews, and the indigestibility of food into this traffic-light system.

What we need now, I believe, is to take a little away from the apparent accuracy of "calorie counting," which seems more scientific, and rethink our traditional diets and eating habits.
This overlaps with what Prof. Dunn has also pointed out, but eating traditional fibrous vegetable dishes, unprocessed meat or fish, dairy products, fermented foods, and whole grain breads, etc., cannot be judged by caloric benefits alone.
Those foods contain far more vitamins and minerals than processed foods, and their fiber content keeps our gut bacteria in good condition, gives us moderate satiety, prevents rapid absorption of glucose, and provides many other health benefits. Depending on how you eat them, it should be possible to lose weight without worrying about caloric intake.
References:
[1]Giles Yeo. Calories on food packets are wrong–it's time to change that. 2021.
[2] Japan Food Research Laboratories. The Energy in Food. 2003.
[3] The Nutrition Coordinating Center (NCC). Primary Energy Sources.
[4] Rob Dunn. Science Reveals Why Calorie Counts Are All Wrong. 2013.
[5]Novotny JA et al. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012 Aug;96(2):296-301.
[6]Westerterp KR. Diet induced thermogenesis. Nutr Metab (Lond). 2004 Aug 18;1(1):5.
[7]Barr SB, Wright JC. Postprandial energy expenditure in whole-food and processed-food meals: implications for daily energy expenditure. Food Nutr Res. 2010 Jul 2;54.
[8]Kazuko Takada. Absorption and Utilization of Energy in the Body. Physical Fitness Science. 2007, Pages 56, 288.
[9]Cynthia Graber, Nicola Twilley. Why the calorie is broken. BBC future. 2016.
[10]Richard Wrangham, Rachel Carmody. Why Most Calorie Counts Are Wrong. Harvard University. 2015.
2022.06.12
Can Thermodynamics Explain Why We Gain Weight?
-
Contents
-
- What does the first law of thermodynamics tell us?
- The human body is a mass of chemical reactions
- Calories eaten is not the same as calories the body takes in. My thoughts
<The bottom line>
First, please refer to the following article.
The Calorie Principle and Weight Gain; The Causality Has Been Obscure
According to Gary Taubes, the author of Why We Get Fat (2010), in the early 1900’s, Carl von Noorden, a German diabetes specialist, first argued that we get fat because we take in more calories than we expend.
This view has persisted to the present day, leading many experts to firmly believe that excessive caloric intake and/or lack of exercise are the primary causes of weight gain[1]. This time, I would like to share the “law of thermodynamics,” which was said to be the basis for that theory. Mainly quoted from the book, it is so interesting that I think it is worth reading.
1. What does the first law of thermodynamics tell us?
"There are three laws of thermodynamics, but the one that the experts believe is determining why we get fat is the first one.
This is also known as the law of energy conservation: all it says is that energy is neither created nor destroyed but can only change from one form to another.
Blow up a stick of dynamite, for instance, and the potential energy contained in the chemical bonds of the nitroglycerin is transformed into heat and the kinetic energy of the explosion.

Because all mass-our fat tissue, our muscles, our bones, our organs, a planet or star, Oprah Winfrey-is composed of energy, another way to say this is that we can't make something out of nothing or nothing out of something.
This is so simple that the problem with how the experts interpret the law begins to become obvious.
All the first law says is that if something gets more or less massive, then more energy or less energy has to enter it than leave it.
It says nothing about why this happens. It says nothing about cause and effect. It doesn't tell us why anything happens; it only tells us what has to happen if that thing does happen. A logician would say that it contains no causal information.(*snip*)

Imagine that, instead of talking about why we get fat, we're talking about why a room gets crowded.
Now the energy we're discussing is contained in entire people rather than just their fat tissue.
Ten people contain so much energy, eleven people contain more, and so on. So what we want to know is why this room is crowded and so overstuffed with energy- that is, people.
If you asked me this question, and I said, Well, because more people entered the room than left it, you'd probably think I was being a wise guy or an idiot. Of course more people entered than left, you'd say. That's obvious. But why? And, in fact, saying that a room gets crowded because more people are entering than leaving it is redundant-saying the same thing in two different ways-and so meaningless.
Now, borrowing the logic of the conventional wisdom of obesity, I want to clarify this point. So I say, Listen, those rooms that have more people enter them than leave them will become more crowded. There's no getting around the laws of thermodynamics. You'd still say, Yes, but so what? Or at least I hope you would, because I still haven't given you any causal information.
This is what happens when thermodynamics is used to conclude that overeating makes us fat. (*snip*)

The National Institutes of Health says on its website, “Obesity occurs when a person consumes more calories from food than he or she burns.”
By using the word “occurs,” the NIH experts are not actually saying that overeating is the cause, only a necessary condition.
They're being technically correct, but now it's up to us to say, Okay, so what? Aren't you going to tell us why obesity occurs, rather than tell us what else happens when it does occur?”
(Gary Taubes. 2011. Why We Get Fat. Pages 73-5.)
2. The human body is a mass of chemical reactions
"The first law states that energy can neither be created nor destroyed. In other words, energy can be converted from one form to another, but the total amount of energy in the universe remains constant. How might this law apply to weight management?
Suppose someone has stable weight over time. The first law dictates that, in theory, the number of calories consumed by this individual in the form of food is equal to the calories the individual expends during metabolism and activity. In other words, 'calories in = calories out’.(*snip*)
However, the first law of thermodynamics actually refers to what are known as ‘closed systems' -ones that can exchange heat and energy with their surroundings, but not matter. Is this true for human beings?

Actually, no: the human body does indeed exchange matter with its surroundings, principally in the form of the food (matter in) and as waste products such as urine and faeces (matter out).
Also, technically speaking, the first law refers to systems in which chemical reactions do not take place.
But the human body is essentially a mass of chemical reactions. So, here again, the first law of thermodynamics cannot apply where weight management is concerned."
(Jone Briffa. 2013. Escape the Diet Trap. Pages 63-4. )
3. Calories eaten is not the same as calories the body takes in. My thoughts
Two authors have made excellent points about the relationship between thermodynamics and weight management. Based on those thoughts, I would also like to mention two points about the relationship between thermodynamics and my theory.
(1)What constitutes "caloric intake"
I also believe that if a person has a stable weight over many years, then the “energy entering the body” and the “energy used within the body” must be balanced.
The issue, however, lies in determining at what point we have "taken in" energy.

If we consider “caloric intake” as calories from food at the point it enters our mouths, then it’s not surprising that for some people, this doesn’t equal the energy expended. This is because, as Dr. Briffa pointed out, our bodies are not "closed systems."
If we consider energy actually absorbed from the gut to be "calories consumed," as gut microbiologists believe the gastrointestinal tract is outside the body, then it should be considered more of a "closed system."
Of course, it’s impossible to calculate each person’s absorption efficiency. Therefore, we currently determine the calorie content of individual foods based on the Atwater coefficient, summing these values to estimate daily caloric intake.
However, we should keep in mind that these are only estimates or approximations. I believe that the actual amount of nutrients and energy absorbed varies with factors such as cooking methods, food digestibility, combination of foods, exercise intensity, and hunger levels,etc.
While von Noorden’s claim that “we get fat because we consume more calories than we expend” is true in a sense, it’s unclear exactly when we can consider energy as being “consumed” by the body.
(2) When energy intake increases
Based on my intestinal starvation mechanism concept, even if a person who has maintained the same weight over the years, significantly reduces their usual caloric intake (e.g. about two thousand kcal daily) and the intake of carbohydrates, but meets the "three factors + one" criteria that cause intestinal starvation, they will gain weight (this means that the set-point weight itself has risen due to an increase in absorption ability).
[Related article] →Three (+one) Factors to Accelerate “Intestinal Starvation”
Of course, weight gain occurs when you return to your original diet afterward. In this case, since absorption efficiency itself has increased compared to before, both body fat and lean tissue contribute to the weight gain.
In short, even though you are eating the same amount of food (calories) as before, you are taking in more energy and nutrients into your body than before, which means you are getting bigger/fatter. In the words of Taubes, "a room crowded with ten people now has eleven people," and in this case, it is “intestinal starvation” that has caused it.
The bottom line
(1)The basis for experts believing that “we gain weight because we consume more calories than we burn” is the law of energy conservation (the first law of thermodynamics).
(2)Since the human body is a mass of chemical reactions and not a "closed system," it does not make sense to compare the total calories actually eaten with the calories expended. In this case, the "first law of thermodynamics" does not hold.
(3)If we base it on the calories actually absorbed in the intestines, it should be closer to a "closed system" and be balanced with the calories expended through one’s basal metabolism and activity,etc.
(4)When intestinal starvation is induced, weight gain can occur even if you are consuming the same amount of calories as before, suggesting an increase in the set-point weight.
In this case, the absorption ability has increased, meaning that more energy and nutrients are taken into the body, so weight gain involves not only body fat but also an increase in lean tissue.
2022.05.22
The Calorie Principle and Weight Gain; The Causality Has Been Obscure
Contents
- The birth of the "calories-in/calories-out" theory
- Obesity is still on the rise
- Carl von Noorden's book
In Japan, most people believe that “taking in too many calories and lack of exercise are the causes of being overweight,” which I believe is largely due to statements made by experts, nutritionists, etc. on television.
When I launched this website in 2014, I wanted to argue against that in my website, but I couldn’t find any academic papers and other resources that showed the "causal relationship between caloric intake and becoming obese."
However, around a year after I started blogging, I came across this great book: “Why We Get Fat” written by Gary Taubes. It is surprising that it was published in Japanese in 2013.
After all, this is the only book I can rely on. In explaining what I want to say, I first needed to let you know that, "the direct cause of being overweight is not determined by overeating.”
1. The birth of the "calories-in/calories-out" theory

"Ever since the early 1900s, when the German diabetes specialist Carl von Noorden first argued that we get fat because we take in more calories than we expend, experts and non-experts alike have insisted that the laws of thermodynamics somehow dictate this to be true.
Arguing to the contrary, that we might actually get fatter for reasons other than the twin sins of overeating and sedentary behavior, or that we might lose fat without consciously eating less and/or exercising more, has invariably been treated as quackery- "emotional and groundless," as the Columbia University physician John Taggart insisted in the 1950s in his introduction to a symposium on obesity. “We have implicit faith in the validity of the first law of thermodynamics," he added.

Such faith is not misplaced. But that does not mean that the laws of thermodynamics have anything more to say about getting fat than any other law of physics.
Newton's laws of motion, Einstein's relativity, the electrostatic laws, quantum mechanics -they all describe properties of the universe we no longer question.
But they don't tell us why we get fat. They say nothing about it, and this is true of the laws of thermodynamics as well.
It is astounding how much bad science-and so bad advice, and a growing obesity problem-has been the result of the experts' failure to understand this one simple fact. The very notion that we get fat because we consume more calories than we expend would not exist without the misapplied belief that the laws of thermodynamics make it true."
(Gary Taubes. Why We Get Fat. New York: Anchor Books, 2011, Pages 72-3.)
(*snip*)
"In 1934, a German pediatrician named Hilde Bruch moved to America, settled in New York city. She was “startled,” as she later wrote, by the number of fat children she saw-”really fat one, not only in the clinics, but also on the streets, subways, and in schools.” This was two decades before the first McDonald's franchises was born, and more to the point, 1934 was in the depths of the Great Depression.
Bruch put in effort in the treatment of obese children. It was hard to avoid, she said, the simple fact that these children had, after all, spent their entire lives trying to eat in moderation and control their weight as directed, and yet they remained obese.

The physicians of Bruch's era weren't thoughtless, and the doctors of today are not, either.
They merely have a flawed belief system-a paradigm-that stipulates that the reason we get fat is clear and incontrovertible, as is the cure.
We get fat, our physicians tell us, because we eat too much and/or move too little, and so the cure is to do the opposite. (*snip*)
▽“The fundamental cause of obesity and overweight," as the World Health Organization says, “is an energy imbalance between calories consumed on one hand, and calories expended on the other hand."

We get fat when we take in more energy than we expend (a positive energy balance, in the scientific terminology), and we get lean when we expend more than we take in (a negative energy balance).
Food is energy, and we measure that energy in the form of calories. So, if we take in more calories than we expend, we get fatter. If we take in fewer calories, we get leaner.
This way of thinking about our weight is so compelling and so pervasive that it is virtually impossible nowadays not to believe it. Even if we have plenty of evidence to the contrary-no matter how much of our lives we've spent consciously trying to eat less and exercise more without success— it's more likely that we'll question our own judgment and our own willpower than we will this notion that our adiposity is determined by how many calories we consume and expend."
(Taubes. Why We Get Fat. Pages 3-6.)
2. Obesity is still on the rise
"Consider the obesity epidemic. Here we are as a population getting fatter and fatter.
Fifty years ago, one in every eight or nine Americans would have been officially considered obese, and today it's one in every three. Two in three are now considered overweight, which means they’re carrying around more weight than the public-health authorities deem to be healthy.

Throughout the decades of this obesity epidemic, the calories-in/calories-out, energy-balance notion has held sway, and so the health officials assume that either we're not paying attention to what they've been telling us -eat less and exercise more-or we just can't help ourselves.
Malcolm Gladwell discussed this paradox in The New Yorker in 1998.
“We have been told that we must not take in more calories than we burn, that we cannot lose weight if we don't exercise consistently," he wrote. “That few of us are able to actually follow this advice is either our fault or the fault of the advice. Medical orthodoxy, naturally, tends toward the former position. Diet books tend toward the latter. Given how often the medical orthodoxy has been wrong in the past, that position is not, on its face, irrational. It's worth finding out whether it is true.”
(Taubes. Why We Get Fat. Pages 7-8.)
(Gary Taubes’s thoughts on the relationship between thermodynamics and weight gain)
"Obesity is not a disorder of energy balance or calories-in/ calories-out or overeating, and thermodynamics has nothing to do with it. If we can't understand this, we'll keep falling back into the conventional thinking about why we get fat, and that's precisely the trap, the century-old quagmire, that we're trying to avoid."
(Taubes. Why We Get Fat. Pages 73.)
3. Carl von Noorden's book
Japanese television programs still continue to show doctors and nutritionists confidently saying, "The cause of weight gain is, of course, overeating or lack of exercise,”which I find disgusting. However, I hope you can see how flimsy and baseless these theories are.
By the way, I obtained Carl von Noorden's book (archive), which is shown at the beginning of the quotation. You can read it at the following link:


2018.10.21
Do Carbohydrates Make Us Fat or Do Too Many Calories?: The Debate Since the 1800's
Contents
- Low carbohydrates go way back
- The reason why doctors couldn’t accept carbohydrate restriction
- Carbohydrates and fat have opposite properties. My thoughts
<The bottom line >
First, as many of you know, even carbohydrates contain four kcal of energy per gram. So, some readers may think, "After all, isn't being overweight ultimately caused by too many calories?"
But if you think, "too many calories are the cause," you should try to reduce the total amount of calories in your overall diet, mostly focusing on fat/oil intake, which has nine kcal per gram.
On the other hand, the argument that “too many carbohydrates cause weight gain” allows you to eat any amount of meat and fatty/oily foods as long as you cut back on carbs.
In this article, I will look back on the historical argument of whether carbohydrates or calories are the cause of weight gain, and at the end of this article, I would like to share my thoughts.
1.Low carbohydrates go way back
In Japan, a low-carb diet was trendy around 2015, but when we look around the world, this way was repeatedly conducted since the 1800’s. Please note that there are many quoted parts. I needed to share this information with you to explain my theory.
"Jean Anthelme Brillat-Savarin was born in 1755. (*snip*) His passion, though, was always food and drink, or what he called the “pleasures of the table.” He began writing down his thoughts on the subject in the 1790s; Brillat-Savarin published them in a book, The Physiology of Taste, in December 1825. (*snip*)
“Tell me what you eat,” Brillat-Savarin memorably wrote, “and I shall tell you what you are." (*snip*)
Over the course of thirty years, he wrote, he had held more than five hundred conversations with dinner companions who were “threatened or afflicted with obesity,” one “fat man” after another, declaring their devotion to bread, rice, pasta, and potatoes. This led Brillat-Savarin to conclude that the roots of obesity were obvious.

The first was a natural predisposition to fatten. “Some people,” he wrote, “in whom the digestive forces manufacture, all things being equal, a greater supply of fat are, as it were, destined to be obese.”
The second was “the starches and flours which man uses as the base of his daily nourishment,” and he added that “starch produces this effect more quickly and surely when it is used with sugar."

This , of course, made the cure obvious as well, ...(*snip*) (Brillat-Savarin wrote) ...”It can be deduced, as an exact consequence, that a more or less rigid abstinence from everything that is starchy or floury will lead to the lessening of weight.” (*snip*)
What Brillat-Savarin wrote in 1825 has been repeated and reinvented numerous times since. Up through the 1960s, it was the conventional wisdom, what our parents or our grandparents instinctively believed to be true."
(Gary Taubes. Why We Get Fat. New York: Anchor Books, 2011, Pages 148-149.)
(*snip*)
"By the time, a French physician and retired military surgeon named Jean-Francois Dancel had come to the same conclusions as his countryman Brillat-Savarin. Dancel presented his thoughts on obesity in 1844 to the French Academy of Sciences and then published a book, Obesity, or Excessive Corpulence: The Various Causes and the Rational Means of a Cure.
Dancel claimed that he could cure obesity “without a single exception” if he could induce his patients to live “chiefly upon meat," and partake “only of a small quantity of other food."
Dancel argued that physicians of his era believed obesity to be incurable because the diets they prescribed to cure it were precisely those that happened to cause it. (*snip*)

“All food which is not flesh ―all food rich in carbon and hydrogen [i.e., carbohydrates] ―must have a tendency to produce fat,” wrote Dancel. (*snip*)
Dancel also noted that carnivorous animals are never fat, whereas herbivores, living exclusively on plants, often are."
(Taubes. Why We Get Fat. Pages 151-2.)
"Until the early years of the twentieth century, physicians typically considered obesity a disease, and a virtually incurable one, against which, as with cancer, it was reasonable to try anything. Inducing patients to eat less and/or exercise more was just one of many treatments that might be considered. (*snip*)
<1950's>
The effects of a carbohydrate-restricted diets were then confirmed in the 1950s by Margaret Ohlson, head of the nutrition department at Michigan State University, and by her student Charlotte Young.
When overweight students were put on conventional semi-starvation diets, Ohlson reported, they lost little weight and “reported a lack of ‘pep’ throughout... [and] they were discouraged because they were always conscious of being hungry.”

When they ate only a few hundred carbohydrate calories a day but plenty of protein and fat, they lost an average of three pounds per week and “reported a feeling of well-being and satisfaction. Hunger between meals was not a problem.”
The reports continued into the 1970s. (*snip*)
The diets were prescribed for obese adults and children, for men and women, and the result were invariably the same. The dieters lost weight with little effort and felt little or no hunger while doing so."
(Taubes. Why We Get Fat. Pages 151, 157-8.)
2.The reason why doctors couldn’t accept carbohydrate restriction
As you can see, by cutting back on carbohydrates and eating more of other foods such as meat and greasy food, the problem of being overweight seems to be solved...but this is where the "calorie principle" comes into play.
"By the 1960s, obesity had come to be perceived as an eating disorder. (*snip*)
Adiposity 101 was discussed in the physiology, endocrinology, and biochemistry journals, but rarely crossed over into the medical journals or the literature on obesity itself.
When it did, as in a lengthy article in The Journal of the American Medical Association in 1963, it was ignored. Few doctors were willing to accept a cure for obesity predicated on the notion that fat people can eat large portions of any food, let alone as much as they want. This simply ran contrary to what had now come to be accepted as the obvious reason why fat people get fat to begin with, that they eat too much.

But there was another problem as well. Health officials had come to believe that dietary fat causes heart disease, and that carbohydrates are what these authorities would come to call “heart-healthy."(*snip*)
After all, if dietary fat causes heart attacks, then a diet that replaces carbohydrates with more fatty foods threatens to kill us, even if it slims us down in the process. As a result, doctors and nutritionists started attacking carbohydrate-restricted diets."
(Taubes. Why We Get Fat. Pages 159-60.)

(*snip*)(In 1965)
"The Times article, 'New Diet Decried by Nutritionists: Dangers Are Seen in Low Carbohydrate Intake,' quoted Harvard's Jean Mayer as claiming that to prescribe carbohydrate-restricted diets to the public was 'the equivalent of mass murder.' (*snip*)
Well, first, as the Times explained, 'It is a medical fact that no dieter can lose weight unless he cuts down on excess calories, either by taking in fewer of them, or by burning them up.' We now know that this is not a medical fact, but the nutritionists didn't in 1965, and most of them still don't.
Second, because these diets restrict carbohydrates, they compensate by allowing more fat. It's the high-fat nature of the diets, the Times explained, that prompted Mayer to make the mass murder accusation."
(Taubes. Why We Get Fat. Page 161.)

3. Carbohydrates and fat have opposite properties. My thoughts
I ‘d like to talk about this controversy.
Several studies have shown that how we combine the three macronutrients (protein, fat, and carbohydrates) in the diet produces different results in body fat accumulation. It is thought that even the same one calorie has different energy used for digestion and absorption, different hormones to stimulate, and different pathways of how the calorie is metabolized in the body.
Of course I think these studies are great, but the point I'd like to add based on my theory is that "carbohydrates and fat are close to having opposite properties in their digestive processes."
First, refined carbohydrates are more easily digested than meats and fats, and the "dilution effect" or "push-out effect" they have makes our digestion go even faster and makes us feel hungrier.
If we eat an unbalanced diet that lacks vegetables, fat, and dairy products,etc., we are ultimately more prone to inducing intestinal starvation.

In contrast, fats and meats are less digestible. The time required for digestion depends on the quantity and type of food consumed, how we prepare food, or individual differences in digestive ability, but it is generally estimated to be 3-4 hours for proteins and 6-8 hours for fats.
In particular, when fat enters the duodenum, cholecystokinin, a hormone that promotes fat digestion and absorption, is secreted.
However, it is said that these hormones also inhibit the function of the stomach and slow down the gastric emptying process, which can cause stomach upset or bloating.
Plus, a diet low in carbohydrates and high in protein and fat sends dense nutrients to the digestive system, which slows overall digestion. As a result, I believe that it suppresses hunger and in turn, causes the absorption rate to decrease.
That is to say, depending on how we structure our diets, there may be a weight-loss effect even with increased caloric intake (for those who can digest protein and fat quickly, the weight-loss effect may be less pronounced) .
The bottom line
(1) From the early 1800’s through the 1960’s, several studies had shown that overweight people could lose weight without difficulty by replacing some carbohydrates in their diet with a lot of meat and fat. By that time, however, obesity was understood as an eating disorder, and this diet method was discussed only in physiology, endocrinology, etc.
(2) From the 1960’s to the late 1970’s, few physicians accepted the idea that fat people could lose weight by eating lots of meat and fat, because it obviously violated the "calorie principle.”
(3) In addition, health experts came to believe that fat in the diet caused heart disease and that carbohydrates were "heart-healthy." As a result, doctors and nutritionists began attacking low-carb diets.
(4) My thoughts: Both sides have a point, but the caloric intake does not determine everything. Different combinations of foods, even with the same calories, have different effects on weight management. In particular, carbohydrates and fat are close to having opposite properties in their digestive processes.
2017.01.15
There is No Meaning in Simply Calculating Calories You Consume
-
Contents
-
- The Atwater coefficient is based on averages
- How we digest food and absorb it differs in each person
- Obesity can be explained by the 'set-point' theory for body weight
<The bottom line>
I’d like to talk about how meaningless it is to calculate the accurate caloric intake for the day, and apply it to weight control to lose weight.
It is said that the caloric values of food that we use to calculate caloric intake are based on the Atwater system(the coefficient of 4 kcal/g for protein and carbohydrate, 9 kcal/g for fat, and 7 kcal/g for alcohol), but various problems have been pointed out regarding the accuracy of these factors.
[Related article]
Calorie Calculation: Why the Atwater Coefficient Is Not Perfect
I believe that one of the problems is that digestibility and absorption rates are based on a system of averages, and I would like to explain that. (This article focuses on how people absorb energy differently, and I won’t mention the process after absorption such as the metabolic process or the differences in hormone secretion, etc.)
1.The Atwater coefficient is based on averages

It is said that, "if you take in more calories than your body needs, you will gain weight, and if you burn more calories than you take in, you will lose weight."(Note 1) This message is very simple, and in a way, it sounds very direct.
However, it is dangerous to accept because the phrases "calories your body needs," and "calories you take in," are ambiguous.
It is said that the calories that the body actually expends including basal metabolism are constantly changing[1], and in my opinion, the calories that the body actually absorbs from food are constantly changing as well.
So, I think it is difficult to derive an accurate value by simply comparing total caloric intake and expenditure.
The Atwater coefficient is based on the average energy value that we can absorb from food, but it seems like it has not been investigated how the absorption rate varies depending on what time of day you eat, how often you eat, or how you combine other foods in a meal.
Also, since this factor is calculated from an average of the subjects, it assumes that everyone has the same digestion and absorption ability[2].
<Note 1>
In 1878, German nutritionist Max Rubner crafted what he called the "isodynamic law". The law claims that the basis of nutrition is the exchange of energy, and was applied to the study of obesity in the early 1900s by Carl von Noorden. (Wikipedia [A calorie is a calorie])
2. How we digest food and absorb it differs in each person
Because I was very thin, my digestibility was poor, and I often had gastrointestinal problems. I felt like I was a guinea pig. If I ate too much fatty or fibrous food, my stomach would be upset even after several hours. And if I forced myself to eat my next meal in that state, I felt that I lost even more weight.
In the Atwater coefficient, fat is said to have a caloric value of 9 kcal/g because its physical combustion heat is 9.4 kilo calories and its digestibility coefficient is set at 95% [3], but for people who have trouble digesting it, including myself, that number is meaningless.
In the following, I will explain how our absorption rate varies for an individual based on my experience.
(I) Absorption rate is not constant but increases from hunger or exercise
Some experiments have shown that when people who want to lose weight reduce their caloric intake and semi-starve themselves, their basal metabolic rate also decreases[1], but I do believe that the absorption rate also changes.
The absorption rate should increase during prolonged periods of hunger or after exercise. This, I believe, is true, not only for energy sources but also for all nutrients, including calcium and other micronutrients.
When you are too hungry and tired, your body is working hard trying to take in more nutrients; if you drink alcohol, you may get pretty intoxicated, or if you eat ramen noodles or sweets, your blood sugar level may spike.

To be more specific, even if you reduce the 700 kcal lunch to 400 kcal and eat nothing until dinner, there is still breakfast in your gut, and your intestines wil be working really hard to take in nutrients out of what is left.
Even when the body uses stored energy sources, not all of them lead to a reduction in body fat because some energy sources are used first. In addition, I believe, the more hunger continues, the higher the absorption ability after dinner is, so the body fat should not be reduced as calculated even if the reduced 300 kcal of energy each day is totaled, for instance, for a two-week period.
(The body is said to be in equilibrium with less weight loss than expected, since the basal metabolism also decreases along with weight loss.)
On the contrary, if you continue to force yourself to eat every three to four hours even though you are not feeling hungry, your absorption rate will decrease, relatively speaking. (Of course, it differs from person to person.)
Consider this for instance, after we eat food, it digests for a few hours, and finally, when it is starting to be absorbed, another food comes into the stomach. So, the body will perceive that, “here comes some more food... we can simply take nutrition from that.”
In the previous example of blood glucose, if you eat ice cream two hours before a meal, the rise in blood glucose levels at the meal should slow down.

While a person doing intense exercise with barbells may need to drink protein and milk between meals, the average person doing light exercise may lose weight from this type of eating because the absorption rate decreases, and energy for digestion (diet-induced thermogenesis) is increased, which can be beneficial for weight loss!
(II) The absorption rate varies depending on the combination of foods
How we combine different foods in a meal also makes a difference in hunger and the absorption rate.
As an example, consider a 400 kcal of breakfast (a slice of toast, ham, fried egg). Even if the caloric intake increases, I think adding a burdock salad, cheese, beans, sautéed mushrooms, etc., to breakfast will suppress hunger at lunch and help reduce the rise in blood glucose levels.
I believe it’s partly because that by constantly eating less digestible foods that contain a lot of fiber, and foods that take longer to digest, undigested food will remain in the gut, which suppresses hunger and decreases the absorption rate. It might also be possible that the fiber itself slightly hinders absorption.

Therefore, even if caloric intake is increased, adding these foods to the three daily meals can still be beneficial for dieting.
Conversely, even if caloric intake is reduced, eating only fiber-poor and highly processed foods which are digested quickly give us a lot of energy for very little work[2]. It may increase the absorption rate because of the constant feeling of hunger, and in turn, it may result in repeated ups and downs in blood sugar levels.
(III) Fat does not always make you fat
How we combine macronutrients such as carbohydrates, proteins, and fats in a meal also makes a difference.
Based on calorie theory, we are taught by nutritionists, doctors, and TV programs, that "if you eat a lot of fat, you will get fat" because fat has nine kilo calories of energy per gram.
However, studies have repeatedly shown until the 1970's that diets that reduced carbohydrates and increased protein and fat as much as one wanted, as seen in carbohydrate-restricted diets, had caused weight loss in subjects, even though their daily caloric intake increased[4].

The DIRECT (Dietary Intervention Randomized Controlled Trial) study conducted in Israel in 2008 also confirmed that the "Mediterranean Diet" and the "Atkins Diet" (low carbohydrate) had a greater effect on body fat loss than the "low-fat diet" [5 ].
Of course, it is known that the energy to digest protein (diet-induced thermogenesis) is higher and that it makes a difference in the hormones stimulated, but what I’d like to add is the absorption factor.
For the same reason as in section (2) above, a diet that reduces easily digestible refined carbohydrates and relatively increases fats and meats (especially unprocessed slabs or chunks) , etc., may help suppress hunger, decrease absorption rates over time, and be effective for dieting, as undigested food will remain in the intestines for longer periods of time. More benefits should be obtained by incorporating fat into each meal or even between meals as snacks.
Of course, this effect may be less pronounced for people or ethnic groups that can digest fat and protein quickly, but for those who cannot digest fat quickly, including myself, consuming more fat will not cause weight gain.
I believe this may be related to data showing that in the U.S., the rates of obesity defined as having a body mass index of thirty or more increased dramatically from 1977 as a result of the promotion of low-fat diets (high carbohydrate) between 1976 and 1996 [6].
[Related article] Eating Fat/Oil Is a Deterrent to Gaining Weight
3.Obesity can be explained by the 'set-point' theory for body weight
The explanation in section 2 above is how the absorption rate changes in an individual, but as I’ve mentioned throughout this blog, the fundamental difference between overweight and thin people can be explained by the difference in 'set-point' for body weight.
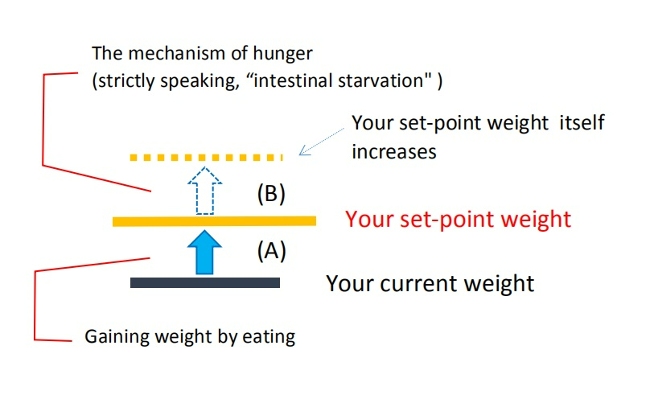
When you gain or lose some weight depending on the amount of calories ingested or burned, I believe it is within the range of [A] ( blue arrow in the figure).
But the increase in set-point weight itself is not directly related to the amount of calories you take in, because it is induced by the intestinal starvation mechanism.
Moreover, according to my theory, having a higher set-point weight is associated with "increased absorption efficiency" compared to a person of average weight. I believe this leads to an overall increase in absorbed nutrients, which means weight gain includes not only body fat but also increased lean mass.
At this point, I can't point out the discrepancy in the Atwater coefficient to explain how one’s set-point for body weight changes, but I would certainly like to prove that people can gain weight due to intestinal starvation.
The bottom line
(1)While it makes sense to calculate daily caloric intake as an average guide in the case of mass food preparation (school lunches, elderly homes, etc.), it does not make much sense to apply it for dieting for each individual. There are many other problems in the concept of being overweight.
(2)The Atwater coefficient is based on the average subject’s ability to digest food , but the process of human digestion and absorption is complex and cannot be described by a system that consist of an average value.
(3)In my opinion, one’s absorption rate varies with how hungry you feel and the intensity of exercise you do. Moreover, what time of the day you eat, meal intervals, and which different foods are combined in a meal also affect the outcome.
(4)I believe that the fundamental cause of being overweight can be explained by the higher set-point weight, and reducing caloric intake may result in temporary weight loss, but in the long run, it will not be effective.
References:
[1] Jason Fung. The Obesity Code. Greystone books. 2016. Page 30.
[2] Rob Dunn. Science Reveals Why Calorie Counts Are All Wrong. 2013.
[3] Japan Food Research Laboratories. The Energy Content of Food. 2003 Jul.
[4] Gary Taubes. Why We Get Fat. 2013. New York: Anchor books. Pages 144-158.
[5]Jason Fung. The Obesity Code. 2016. Pages 100-1.
[6]Jason Fung. The Obesity Code. 2016. Pages 19-20.
